ARLINGTON, Va. – With freshman Nicole Fu pacing the squad at a 2-0 singles mark on the weekend,…
Blog
-

Diagnostic Features and Prescription Rules of Influenza-like Illnesses
Introduction
Influenza, commonly known as the flu, is an acute respiratory infection caused by influenza viruses,1 characterized by sudden onset, rapid transmission, and the potential to reach epidemic levels. Influenza-like illness (ILI) is a…
Continue Reading
-
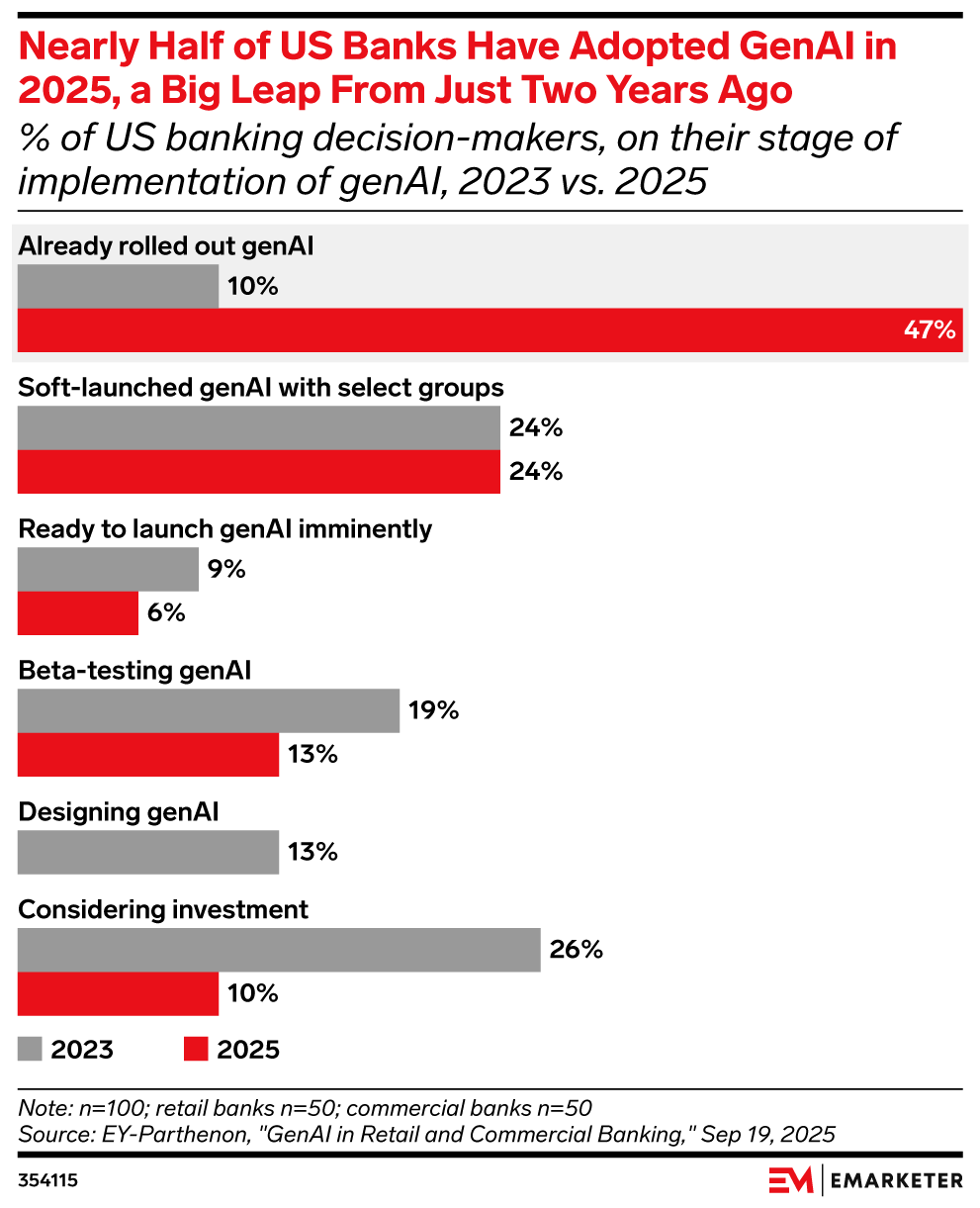
Nearly half of US banks have rolled out genAI in 2025
Key stat: 47% of US banking decision-makers say their institutions have already will have fully rolled out generative AI, up from 10% in 2023, said data from EY-Parthenon.
Beyond the chart:
- Over two-thirds (67%) of senior banking executives reported increased investment in genAI over last year, found a March Capgemini survey.
- However, 56% of US debit card holders say human oversight is very important to help resolve disputed transactions and 55% say the same about handling customer service issues, as noted in a June survey from Auriemma Group.
Use this chart: This is the time to move from pilot projects to full deployment. Laggards risk falling behind in customer experience, cost savings, and innovation. Strategy teams should benchmark where they stand against peers and identify quick-win use cases (like chatbots or risk modeling) to accelerate adoption.
Related EMARKETER reports:
Methodology: Data is from the July 2025 EY-Parthenon report titled “GenAI in Retail and Commercial Banking.” 100 US banking employees were surveyed during March 2025. The sample included 50 respondents from retail banks and 50 from commercial banks, all with direct involvement in or knowledge of genAI initiatives. Respondents held roles in client servicing, marketing, onboarding, product strategy, investment, or technology. Titles included C-level executives and heads of departments tied to genAI applications such as ChatGPT, DALL-E, OpenAI, and Microsoft Azure.
Continue Reading
-
A liver extracted from a genetically modified pig transplanted into a 71-year-old man – Genetic Literacy Project
- A liver extracted from a genetically modified pig transplanted into a 71-year-old man Genetic Literacy Project
- First gene-edited pig liver transplanted into a living person statnews.com
- In a First, Pig Liver Helped a Cancer Patient Survive for…
Continue Reading
-

HOW TO WATCH Steelers vs. Bengals for Week 7 of the 2025 Season – Bengals.com
- HOW TO WATCH Steelers vs. Bengals for Week 7 of the 2025 Season Bengals.com
- NFL Expert Picks Week 7: Bengals Cover on TNF. BetMGM Sportsbook
- Steelers Favored in Kill or Be Killed Matchup Against Bengals BVM Sports
- Ja’Marr Chase Says Steelers Are…
Continue Reading
-

Consistency in the Chaos: FDA Approvals Within Average Range as Q4 Kicks Off
A chaotic nine months at the FDA has witnessed the toppling of more than half the agency’s senior leadership, the axing of 3,500 more staff and a safety scandal that upended the gene therapy sector, which was followed by the subsequent ouster—and surprising return—of CBER Director Vinay Prasad. But the first three quarters of 2025 have seen an average number of new drug approvals, according to an analysis by Jefferies.
“Fears of a dysfunctional FDA may be overblown,” the analysts wrote to investors on Aug. 25. “We think the FDA’s actions YTD have not signaled a more stringent or irrational agency.”
The Jefferies group counted 28 total approvals as of that date between the Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) combined and extrapolated out to 43 by the end of 2025, a number that would fit squarely into the range of 37–68 over the past 5 years.
“The bottom line is, we didn’t sense anything drastically alarming or different compared to prior years,” Jefferies analyst Andrew Tsai told BioSpace in an interview last month.
As for rejected drug applications, these were trending lower than last year, Jefferies reported Aug. 25, thanks to CDER being on pace to issue fewer CRLs compared with the past five years. CBER, on the other hand, was heading toward issuing more CRLs.
Rare Diseases on Track
The acceleration of therapies for rare diseases to the market has been a top-stated priority of FDA Commissioner Marty Makary since he took office in April. Makary first floated the idea of a conditional approval pathway for such therapies in one of his first media interviews, and the FDA has since rolled out the Rare Disease Evidence Principles, a new framework meant to streamline the approval of drugs for ultra-rare diseases.
“They seem to be following it through with what they want to achieve, especially within the rare disease side, and expediting drugs and so forth,” Tsai said.
This year, the regulator has approved 14 novel therapies for rare diseases—including ones for alkaptonuria, recessive dystrophic epidermolysis bullosa and phenylketonuria. On the other hand, seven others received complete response letters, some of which came as quite a surprise to the company executives and other experts.
Capricor Therapeutics CEO Linda Marbán was caught off-guard after the company’s cell therapy for Duchenne muscular dystrophy was rejected in July, saying on Capricor’s second quarter earnings call the next month, “The complete response letter was unexpected given the trajectory of positive interactions [with the FDA].” And 22 scientists who designed and ran the trials of Replimune’s advanced melanoma drug, RP1, wrote an open letter urging the FDA to reconsider its July rejection.
Stealth BioTherapeutics has had success getting the FDA to reconsider its May rejection of Barth syndrome drug Forzinity. After refiling in August, the company was granted an expedited review and won accelerated approval less than a month later.
The highest number of novel drug approvals this year have come from the oncology space, according to data collected by both BioSpace and Jefferies. Treatments for infectious diseases and autoimmune/immunological diseases are also high among this year’s FDA approvals.
New Admin, New Party
In the past 25 years, control of the White House has jumped back and forth from Republican to Democrat multiple times. During three of those changeovers, a new FDA commissioner was installed during the administration’s first year. As drug approvals are not political decisions, “You would not expect a flood of new drug approvals in the inaugural year of a new administration,” suggested Steven Grossman, policy and regulatory consultant and author of the FDA Matters blog.
This has proven to be the case, Grossman said, except in 2017, during President Trump’s first term. That year, when Scott Gottlieb took the reins from Robert Califf, who served as commissioner during Barack Obama’s second term, the FDA approved 46 new drugs—more than double the number greenlit the previous year.
“The last two years of an eight-year administration are not known for policymaking or starting new initiatives,” Grossman told BioSpace. “I would not expect that to have slowed drug approvals in 2015 or 2016 or led to a surge of approvals in 2017.”
Indeed, in 2009, when Margaret Hamburg took the helm at the FDA during Obama’s first year in office, the agency approved just one more novel drug than in 2008. The results of this year’s changeover—from Califf, who served a second stint as commissioner, to Makary—are still-to-be-determined.
Potentially adding a new wrinkle to future data analysis attempts is the current U.S. government shutdown. The FDA announced on Oct. 1 that it would be unable to accept any new drug applications until after the shutdown is resolved—as to do so would require the agency to accept industry user fees assessed for 2026, which it is unable to do during this “lapse period,” Leerink Partners explained in a note to investors that morning.
Only time will tell if this has any bearing on 2025’s ultimate approval tally.
New analysis from Jefferies shows that rare disease and cancer drugs granted the status are especially likely to be approved.
Continue Reading
-

More Evidence Emerges That One of Saturn’s Moons Could Harbor Life
A recent study of Enceladus, one of Saturn’s moons, has detected several organic compounds that had never been recorded there before. The findings, published this month in Nature Astronomy, provide new clues about the interior chemical…
Continue Reading
-

Connecting neural activity, perception in the visual system
Lindsay points toward an important issue: the disconnect between research on the sensory processing of complex, natural visual information and…
Continue Reading
-

Xe3P architecture coming to Intel’s next Arc GPUs in roadmap update
Intel has confirmed it is developing a “Next ARC Family” of GPUs, utilizing its “Xe3P” graphics architecture. This news, revealed at the 2025 Tech Tour US, follows an in-depth look at Intel’s Xe3 GPU architecture, which is…
Continue Reading
-

Safer, More Sustainable Antimicrobials To Prevent Infection Of Cow Udders
Image: (From left) Prof Mary Chan from Nanyang Technological University, Singapore, Dr Kaixi Zhang from the Singapore-MIT Alliance for Research and Technology, and Prof Paula Hammond from the Massachusetts Institute of Technology were…
Continue Reading
